How Hydrogen Water Is Made (Beginner’s Guide)
Electrolysis, magnesium reactions, and gas infusion—what each does, how they differ, and how to choose the right option.

Hydrogen water is regular water with additional dissolved molecular hydrogen (H₂). You can make it at home or buy it pre-made. The production method and storage determine how much dissolved hydrogen you actually drink.
Key Takeaways
- Three core methods: electrolysis, gas infusion, and magnesium reactions.
- Concentration (ppm) varies by method, device quality, and storage.
- Make it at home (bottles, machines, tablets) or buy ready-to-drink.
New to H₂? Start with our Hydrogen Water: The Ultimate Guide (2025).
Bottom line: Choose the approach that fits your routine and target ppm—then store it properly to retain dissolved H₂.
How Hydrogen Water Is Made
Hydrogen water production relies on three practical approaches that add molecular hydrogen to regular water.
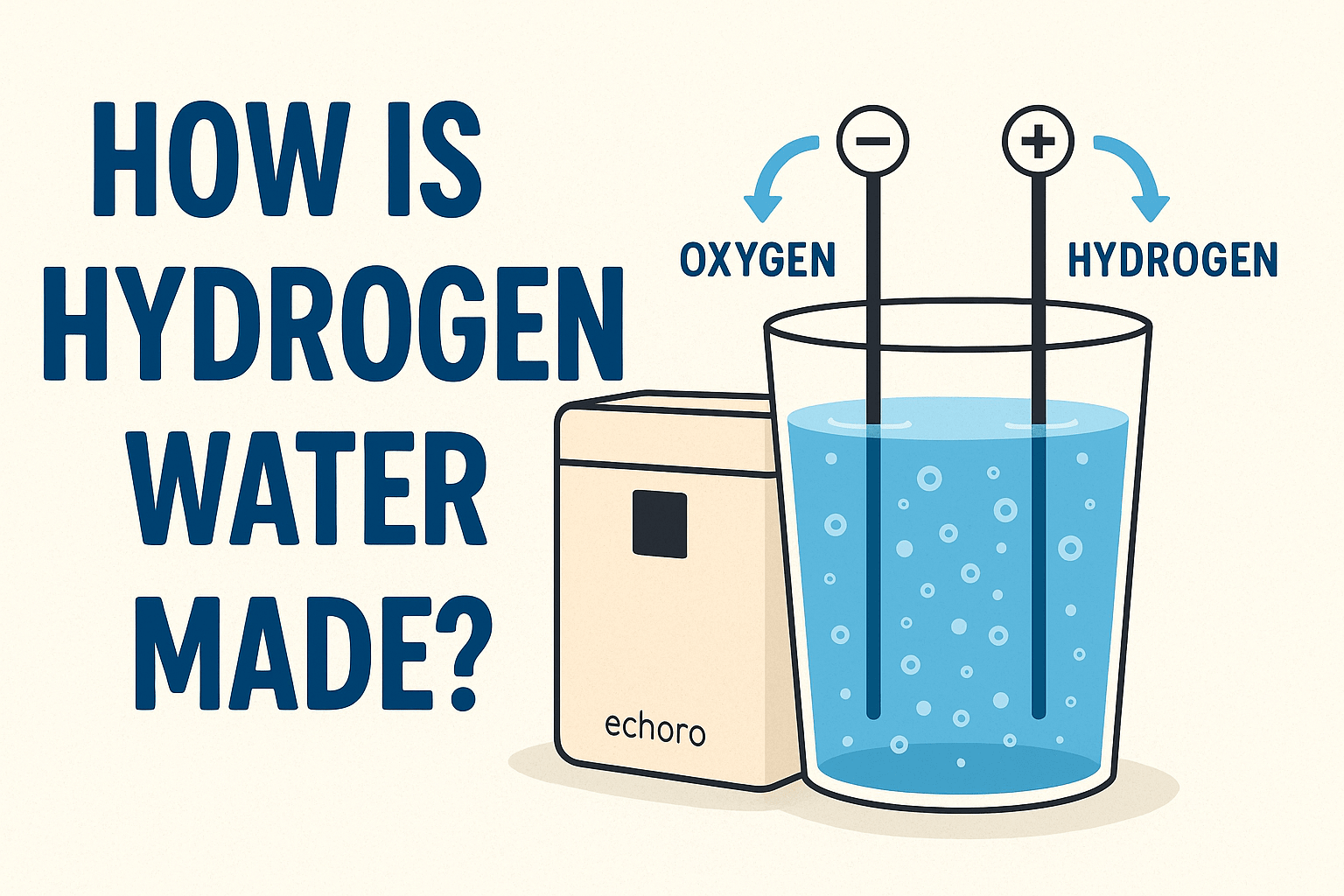
Electrolysis (SPE/PEM)
Applies electric current across membranes to generate and dissolve H₂ into water.
Magnesium Reaction
Magnesium + water → hydrogen gas; common in tablets or sticks.
Gas Infusion (Industrial)
High-pressure injection and nano-bubble tech for canned/bottled H₂ water.
Electrolysis Method
How it works: An electrical current splits H₂O into hydrogen and oxygen. Devices use SPE/PEM membranes and vents to keep unwanted gases out of the final water.
- Typical cycle: ~3–5 minutes
- Typical concentration: ~0.5–1.6 ppm (mode/water dependent)
- Formats: bottles, countertop, under-sink
Core Components
- Platinum/titanium electrodes
- PEM membrane & gas separation
- Water chamber & power supply
Echo Ultimate (premium) · Echo H2 (countertop) · Echo Flow (under-sink)
Magnesium Reaction Method
Elemental magnesium reacts with water to produce hydrogen gas: Mg + 2H₂O → Mg(OH)₂ + H₂.
Pros: portable, no electricity, budget-friendly. Note: by-product Mg(OH)₂ raises pH slightly.
What to Expect
- Dissolve time: ~10–30 minutes
- Typical ppm: ~1–3 (tablet/volume dependent)
- Taste/ingredients vary by brand
Industrial Production Processes
- Pressure injection: H₂ forced into water at ~2–5 ATA
- Membrane electrolysis: scaled, controlled gas separation
- Nano-bubbles: ultra-small bubbles to aid dissolution
Why it matters: tighter control over concentration, purity, and packaging. Often achieves higher ppm (~3–5) than most home setups.
Common formats: aluminum cans and specialized bottles for retail/gyms.
Factors Impacting Hydrogen Water Quality

Purity of Source Water
Start with clean, filtered water—chlorine/chloramines and other contaminants can interfere with hydrogen dissolution and retention.
- Remove: chlorine/chloramines, heavy metals, sediments, organics
- Mind minerals & pH (device requirements vary)
Hydrogen Concentration (PPM)
PPM depends on method efficiency, device quality/maintenance, temperature, and storage time.
- Keep water cool; cap promptly
- Minimize agitation & headspace
- Maintain membranes/filters on schedule
Learn more: How long H₂ lasts · Storage tips

